
These rays are electrons which are produced from the gas ionization inside the tube. The electrons which are ejected from gas ionization travel towards the anode. So, these rays strike and ionize the gas sample present inside the container.

How were cathode rays produced using a discharge tube?Ĭathode rays come out from the cathode as the cathode is charged negatively. 01 mm of mercury), the gases become good conductors of electricity and begin to flow in the form of rays which are cathode rays. When a high voltage charge from an induction coil is applied to tubes filled with gases at very low pressure (0. What are cathode rays How are these rays formed? As they are energetic electrons, when they strike a certain substance or the glass wall of the discharge tube, this excites the atoms of the substance or the glass and cause them to emit light, a glow called fluorescence. Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.” Why do cathode rays produce fluorescence?Ĭathode rays produce fluorescence in some materials. Electrons were first discovered as the constituents of cathode rays. How did JJ Thomson used the cathode ray tube?Ĭathode rays carry electronic currents through the tube.

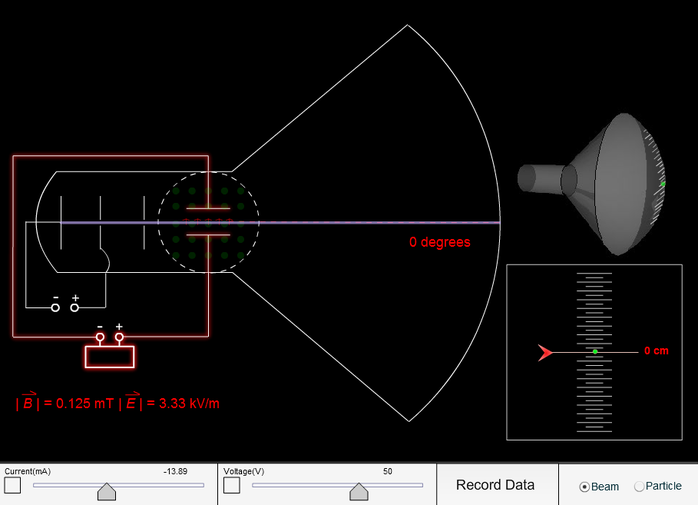
During his experiment he discovered electron and it is one of the most important discoveries in the history of physics. Thomson experimenting with cathode ray tubes. He deduced that the cathode rays were made up of negatively-charged particles.Ĭathode ray experiment was a result of English physicists named J. In the second experiment, he discovered that the charge in the cathode rays was negative. This experiment took place in the year 1897.In Thomson’s first experiment, he discovered that cathode rays and the charge they deposited were intrinsically linked together. During cathode ray tube experiment, a negatively charged particle was discovered by J.J. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier. This model explained the description of an inner structure of the atom theoretically.Thomson ‘s experiments, the existence of these particles was not fully known. Thomson ‘s Cathode Ray Tube (CRT): Definition, Experiment & Diagram … Prior to physicist J.J. Thomson ‘s experiment and the charge-to-mass ratio of the electron – WAEC TUTORIALS MaWAEC TUTORIALS The first is the experiment of Joseph John Thomson, who first demonstrated that atoms are actually composed of aggregates of charged pa… Thomson’s Cathode Ray Tube (CRT): Definition, Experiment & Diagra , JJ Thomson’s Experiments with Cathode Ray Tubes, J.J. In his first experiment, JJ Thomson built a cathode ray tube with a metal cylinder on. Experiment 1: JJ Thomsons objective in his first experiment was to prove that the rays emitted from the cathode were inseparable from their negative charge.His model stated: – An atom resembles a sphere of positive charge with negative charge present inside the sphere. After the Cathode ray tube experiment, Thomson gave one of the first atomic models including the newly discovered particle. Thomson built a cathode ray tube by putting two cylinders together and sending a voltage through them. It is a vacuum sealed tube with a cathode and anode on one side. He did this using a cathode ray tube or CRT. Thomson ‘s cathode ray experiment was a set of three experiments that assisted in discovering electrons. In this lesson learn what a cathode ray tube is and how J.J. Thomson ‘s cathode ray tube experiments led to a very important scientific discovery, the electron.


 0 kommentar(er)
0 kommentar(er)
